Air Pollution — A Growing Urban Problem
PAS Report 79
Historic PAS Report Series
Welcome to the American Planning Association's historical archive of PAS Reports from the 1950s and 1960s, offering glimpses into planning issues of yesteryear.
Use the search above to find current APA content on planning topics and trends of today.

|
AMERICAN SOCIETY OF PLANNING OFFICIALS 1313 EAST 60TH STREET — CHICAGO 37 ILLINOIS |
|
| Information Report No. 79 | October 1955 |
Air Pollution — A Growing Urban Problem
Download original report (pdf)
Five years ago PLANNING ADVISORY SERVICE issued an Information Report on Air Pollution Control.1 Since that time much has happened. The federal government this year for the first time appropriated funds for research and technical assistance in air pollution. Private foundations have engaged in intensive research along many related lines and have come up with some unexpected findings. From England has come the new idea of smokeless and semi-smokeless zones; in the United States the idea of performance standards in industrial zoning as a means of controlling air pollution has been gaining momentum.
The gains in knowledge and regulation have been offset in part by a rise in the number of sources of contamination. Air pollution is a byproduct of industrialization and urbanization. In the past five years the petrochemical industry has come of age, the chemical industry is growing at a faster rate than the rate of all industries, and the number of motor vehicles has increased 26 per cent in the last five years.
There is no reason to think that the sources of contamination will diminish in the future and every reason to think they will grow in number and kind and concentration. Uninvestigated and uncontrolled, the situation will get worse as there are more people, more industry, and more gasoline engines.
Air, like water, is being recognized belatedly as a limited resource. There is a limit to the amount of man-made materials it can absorb and still sustain life. This limit was reached in the Meuse Valley of Belgium in 1930, in Donora, Pennsylvania in 1948, Poza Rica, Mexico in 1950, and in London in 1952.
There are also limits to the amount and kind of contaminants the atmosphere can absorb and still be suitable for living in. One of the big issues in air pollution control is the determination of these suitable limits. The vast range of contamination lying between the extremes of undetectable emissions and toxic levels is the area of scientific research and governmental regulation.
Five years ago one of the chief concerns was the measurement of particulate matter — smoke, soot, fly ash, and dust. Today increased attention is being paid to materials that are measured mainly by their effects on structural materials, painted surfaces, vegetation, livestock, and the human eye and respiratory system.
Temperature inversion, which prevents smog from escaping in the Los Angeles basin, is no longer considered a unique circumstance but one that occurs in many parts of the country. Wherever it occurs, it aggravates man-made contamination of the atmosphere.
Thus air pollution is no longer a problem limited to the great refining centers and coal-iron areas. It is likely to be found in any industrial or urban area.
The Present-Day Problem of Air Pollution
It may seem strange that a definition of air pollution is still of interest at this stage in control. However, certain developments have combined to make the problem of air pollution essentially different today from what it was ten or even five years ago.
First, the techniques of controlling smoke emission have been pretty well mastered. Some urban areas are still being blanketed with smoke particles, especially in the winter months. Laws and their enforcement are lacking in such cases, rather than knowledge.
Second, the chemical industry has expanded tremendously. Noxious gaseous by-products, though often visible, are not usually smoke producers in the old sense of the words.
Third, we have become more skeptical and sophisticated about the nature of air pollution. Sources once considered blameless are now suspect. We have learned that inherent in new industrial processes and products is the possibility of unpredicted air contamination. We have learned also that certain products combine with natural elements and with each other to form new compounds that may be far worse pollutants than any isolated emission.
Hence, the meaning of air pollution has broadened. "Air pollution is the general term applied to atmospheric nuisances produced by contaminating substances," according to one study. "The presence of foreign substances in the air is called air pollution," says another.
The shift in emphasis from smoke to other kinds of pollutants is reflected in the changed name of the Air Pollution Control Association — its present official title. A national clearing house for information on all aspects of air pollution control (4400 Fifth Avenue, Pittsburgh 13), this organization started out in 1907 as the Smoke Prevention Association of America. It was later named the Air Pollution and Smoke Prevention Association of America and recently renamed with the title it now bears. National Smoke Abatement week, which it sponsored, is now called Cleaner Air Week.
Recently formed regional societies, such as the Midwestern Air Pollution Prevention Association, Inc., and the Southern California Air Pollution Foundation, likewise are concerned with the varied manifestations of air pollution rather than with the single one of smoke.
In general, then, air pollution can be divided into two main types: (1) smoke, and (2) fumes and gases, etc. Although an oversimplification, this division is useful for purposes of reporting on progress in air pollution control and identifying the kinds of specific problems that urban areas will face in the future.
Smoke
The chief source of smoke is fuel used for domestic and industrial heat and power generation. Smoke represents the products of both complete and incomplete combustion. It is the latter that most often produces the visible smoke that ordinances seek to abate.
The report of the Joint State Government Commission to the general assembly of Pennsylvania in 1951 points out that a division of smoke into its visible and invisible parts is required if the problems of smoke control are to be understood:
Visible Products of Combustion
The components of visible smoke are largely soot and fly ash. Soot represents the carbon particles generally agglomerated with tarry material and is black in color. Fly ash is the noncombustible material found in solid fuels and is generally gray to white in color, depending on the composition of the fuel. Cinders are the larger portions of fly ash and are non-combustible constituents of coal. The solid or visible particles in smoke may be classified as: (1) grit which can be collected by deposit from the atmosphere and (2) particles so small they may stay suspended in air almost indefinitely.
Invisible Products of Combustion
The invisible products of combustion are:
1. Carbon dioxide, which results from complete combustion.
2. Carbon monoxide, which is produced by incomplete combustion.
3. Volatile hydrocarbons (gaseous chemical compounds of carbon and hydrogen), i.e., the combustible gases in fuel which are distilled from the fuel but are not consumed because the temperature of the combustion chamber is not sufficiently high, and which are consequently vented as gases into the chimney.
4. Gaseous combustion products of sulphur, (a) Sulphur dioxide and (b) Sulphur trioxide, which combine with the moisture of the atmosphere to form sulphurous and sulphuric acids.
5. Ammonia in some fuels.
The visible products of combustion, soot, and fly ash, are generally considered the more objectionable and it is principally against them that smoke control ordinances have been drawn. Of the invisible combustion products, the most objectionable are the gaseous combustion products of sulphur which combine with atmospheric moisture to produce sulphurous and sulphuric acids. These acids are corrosive to most materials used in building construction as well as harmful to vegetation and animal fibers.
The successful pioneering attacks on smoke made in St. Louis and Pittsburgh followed two complementary courses: control of fuel and control of equipment. Smoke nuisance was found to vary with the amount of volatile matter in fuel. Although poorly operated gas or oil furnaces may produce a smoke as black as that produced by coal, the fluid fuels are more uniform in character and hence more easily handled. Coal burning, therefore, was the chief problem, coal being highly variable in the components that affect its combustion.
The following table, taken from the Pennsylvania report cited above, shows the differences among some of the more commonly used coals.
| Fixed Carbon Content | Volatile Matter | |
|---|---|---|
| (1) Meta-Anthracite | 98% or more | 2% or less |
| (2) Anthracite | 92% to 98% | 8% to 2% |
| (3) Semi-Anthracite | 86% to 92% | 14% to 8% |
| (4) Low-Volatile Bituminous | 78% or more | 22% or less |
| (5) Medium-Volatile Bituminous | 69% to 78% | 31% to 22% |
| (6) High-Volatile Bituminous | Less than 69% | More than 31% |
Source: Typical Analyses of Coals of the U.S., U.S. Department of Interior, Bureau of Mines, Bulletin 446, p. 13.
The St. Louis smoke control code followed these principles: (1) persons burning high-volatile fuel must use mechanical fuel burning equipment; (2) persons not wishing to use mechanical equipment must use a smokeless fuel.
Any fuel containing less than 23 per cent volatile matter was defined as a smokeless fuel. The ordinance also requires the use of washed coal to eliminate some of the sulfur and fly ash. The plans for fuel burning equipment are approved by the St. Louis Division of Smoke Regulation., which issues a permit for installation. When completed, the equipment is inspected and a certificate of operation issued.
In Pittsburgh, the use or sale of solid fuel with a volatile content higher than 20 per cent is forbidden. However, fuels higher than 20 per cent are permitted if they are of an anti-smoke type, if used in mechanically-fed furnaces, and if approved by the Pittsburgh Bureau of Smoke Prevention.
Government agencies in other eastern and midwestern metropolitan areas have conducted smoke control programs with varying degrees of success, some of which were described in Information Report No. 20. The St. Louis and Pittsburgh ordinances have been emphasized here because of the simplicity of their concept, which strikes directly at the heart of the smoke problem — fuel and equipment — and also because these cities have demonstrated that smoke can be controlled reasonably and effectively.
Similar in some respects to these programs is the Clean Air Bill introduced in the British Parliament in July 1955 and expected to become law within a few months. It, too, is based on the principle that smoke at its source must be prohibited. Under its terms. local authorities would be empowered to establish, subject to ministerial approval, "smokeless zones" and "smoke control areas."
In a smokeless zone (an idea first introduced in Manchester in 1935) the emission of smoke from any source is prohibited. Because low-volatile coals are scarce in England, gas and electricity expensive, and all oil imported, absolute smokelessness throughout an urban area would be difficult to achieve. Therefore, the idea of a smoke control area was devised where the aim is an 80 per cent overall reduction of total smoke. The government believes that this would be within the reach of all industrial plants, except a few using special and difficult processes.
A unique feature of the Clean Air Bill is that part of the cost to individuals — up to 70 per cent of approved conversion expenditure in some cases — would be defrayed by national grants. No payments would be made for houses built after the bill becomes law.
This section has only touched upon smoke control programs which, in matter of fact, are exceedingly complex. Consider, for example, the Allegheny County smoke control ordinance, which contains ten sections on the operation of steel mill equipment alone. Or the matter of standards for determining the extent of the smoke nuisance and the relative merits of the Ringelmann Chart compared with other devices. And finally, the highly important matters of public information and industry cooperation. These aspects and others are treated in references listed at the end of this report.
Fumes, Gases, and Other Pollutants
The most important gaseous contaminants are hydrocarbons, aldehydes, organic acids, oxides of nitrogen, and carbon monoxide. The chief sources of contaminating substances in urban and industrial areas are petroleum refineries, petroleum storage and marketing facilities, internal combustion engines, chemical plants, metals industries, incinerators, burning refuse piles, mineral and earth processing industries, power plants, and homes heated by gas and oil. In agricultural regions the primary sources of pollution are the oxidized hydrocarbons emanating from petroleum processing, transportation, and consumption that spill out into farm areas adjoining urban and industrial areas.
This paragraph, taken from Air Pollution Control (University of California) nicely summarizes the growing portion of the present-day problem of air pollution — contaminants that come not from the burning of coal but from a myriad of combustion and evaporation processes. It also illustrates the point emphasized by Richard F. Hansen (speaking on statutory regulation of air pollution at the United States Technical Conference on Air Pollution, 1952) that smoke results from the same simple operation repeated daily in millions of houses, offices, buildings, and factories throughout the world. By contrast, gaseous pollutants arise from thousands of processes employing divergent types of equipment and techniques.
In spite of the complexity of the problem of pollution by fumes, gases, mists, acids, and vapors, certain sources have been singled out as major contributors, such as motor vehicles, petroleum refineries, and refuse dumps. Moreover, it has been found that individual offenders within any one of these (and other) groups contribute to air pollution in a nearly identical manner. For example, motor vehicles emit the same hydrocarbons in similar quantities; petroleum refineries that employ the same processes emit the same gaseous by-products; and refuse dumps burn in such a way as to produce characteristic fumes and gases.
Therefore, to determine the causes of air pollution it is not necessary to isolate all of the individual members of a group that contribute to it. Nor is it probably necessary in the beginning to identify all of the classes of pollutant contributors. The main problem is to identify the major contributing groups and to find out what operations result in the emission of contaminants. Thus, the problem or air pollution by gases, vapors, mists, etc., can be narrowed down.
Although discussion of air pollution by gases, fumes. and other similar pollutants compose most of the balance of this report, concentration on this type of pollution is not intended to minimize the problem of smoke pollution that still exists in many parts of the country.2 However, since the techniques and equipment for abatement of smoke at the source are available and effective, it is believed that the bigger problem now is air pollution from combustion of materials other than coal.
The extent, duration, and intensity of air pollution in the Los Angeles basin and the resulting large-scale investigations of causes and cures has made available considerable information on the subject, much of which is covered in this report. The availability of material on a local problem does not alone justify its use here. It is justified, however, on the basis of the belief of many experts that what is happening in Southern California could, and well may, happen elsewhere.
AIR POLLUTION AND HEALTH
Two noteworthy advances have been made in the last five years in the field of air pollution and health. One is the development of a universal opinion that general air pollution produces harmful effects on health. Previously, interest in such effects had centered around air pollution disasters, such as Donora and London. Now, levels of air contamination less than those known to directly cause hospitalization and death are believed to be undesirable and possibly dangerous.
The second advance — causally related to the first (though it is hard to say which is cause and which effect) — is medical research on the specific effects of general air pollution on health. Public health officials are investigating air pollution as an environmental health hazard and medical scientists are investigating the effects of specific contaminants. Both kinds of investigation are in the beginning stages, although some tentative findings have been published.
Short-Range Effects on Health
In 1950, the Los Angeles County Medical Association circulated a questionnaire among its members seeking their views on the health effects of air pollution. Out of 4,700 questioned, 2,803 responded. Overwhelmingly, the physicians agreed that Los Angeles smog causes eye and nose symptoms; the majority believed it affects the throat and lungs; and some believed that other parts of the body are affected. It was generally believed that eye and nose irritations from smog are temporary. However, there are no quantitative data for any of these findings.
A survey of the reactions of students and teachers to the extended smog episode of 1954 resulted in the following kinds of answers (Source: Clean Air for California): "All schools within the heavy smog area report a substantially increased incidence of headaches, coughs, eyestrain, excessive nasal discharge, and similar troubles." And, "Possibly even more serious is the effect of the smog upon the behavior of the pupils. Schools report increased restlessness, with more playground fights and other turmoil. Children are irritable, and teachers are discontented and frustrated at having to work in an atmosphere of smog, and the fear of what it may be doing to all of us."
Various investigations to determine whether there is a correlation between smog periods and sickness, absenteeism, and death have been made in the Los Angeles area. However, positive correlations have not been convincingly demonstrated.
In November 1954, the California State Department of Public Health conducted a statewide survey of illness. Sample populations in the Los Angeles area did not show a higher incidence of illness during or immediately after the October 1954 smog. Other data showed no remarkable deviation in the average number of hospital admissions due to diseases of the heart and respiratory system during the period of air pollution. Nor did absenteeism in five manufacturing firms and public utility companies employing a total of about 6,500 persons increase significantly during smog periods. Similarly, no correlation was demonstrated between smog periods and deaths from selected causes, or infant deaths, or deaths in nursing homes, with the exception of an unusually large number of deaths in nursing homes in the week immediately following the heavy smog of late October 1954.
California State Department of Public Health officials point out that these investigations covered a limited period of time and that the data are limited. They say that failure of the data to reveal any measurable smog effects on health does not necessarily mean that no such effects occurred. "Failure to demonstrate it may mean only that the methods of measurement were too crude," they explained.
Examination of the relationship between atmospheric pollution, as determined by sootfall, and respiratory disease death rates in various districts of Cincinnati and Pittsburgh revealed that mortality due to pneumonia and tuberculosis was in direct ratio to the amount of atmospheric pollution. (These tests were conducted by Dr. Clarence A. Mills, professor of Experimental Medicine at the University of Cincinnati in 1946. References to published results are given in the bibliographical sources listed at the end of this report.) A close relationship was also found to exist between pneumonia rates and family income, the amount spent on housing and the extent of overcrowding. However, the incidence of pneumonia in males was twice that of females, the explanation being that at work men are more frequently exposed to polluted air.
Dr. Mills, in a paper entitled, "Public Health Aspects of Air Pollution" (Final Summary Report of Assembly Interim Committee on Air and Water Pollution, State of California, 1951), refers to statistical studies of respiratory deaths in Chicago:
Based on the low rates prevailing in its clean suburbs, Chicago each year has an excess of 258 deaths from pneumonia among white males in its dirtier districts, 241 from tuberculosis and 69 from buccal and respiratory tract cancers — a total of 568 deaths among white males each year from these three respiratory diseases alone in excess of the death rates these diseases show in the city's cleanest districts. Add to this one-tenth as many deaths for white females and for Negro males, and a grand total is obtained of roughly 700 deaths each year, which represents a measure of the respiratory hazard of living in Chicago's dirtier districts.
Long-Range Effects on Health
It has been known for some time that exposure over a long period to concentrations of certain industrial fumes and dusts can cause serious and even fatal conditions to human beings. However, only recently has thought been given to the possible long-range effects of general air pollution. In the words of a report made by the California State Department of Public Health, "Whether there are more subtle and serious effects on the human organism from this type of air pollution [smog] no one can yet say on the basis of present scientific evidence."
However, the American Cancer Society has taken a stronger position:
We are creating a marked cancer hazard in the air over our big cities by dumping all manner of fumes and gases into the atmosphere. The increasing frequency of lung cancer in cities as compared with rural areas all over the world indicates that the atmosphere may be the principal cause of this disease.
(Source: New York Times editorial, November 24, 1953, quoted in Air Pollution Control, The Bureau of Public Administration, University of California, January 1955)
Even more startling is the possibility now being investigated that carcinogenic air pollutants may affect not only parts of the respiratory tract but other parts of the body, such as skin, bones, bone marrow, and bladder — depending on the nature of the agent and the type of exposure.
Chronic effects of air pollution on health do not lend, themselves to the cause-effect kind of analysis that is possible with short-range toxic effects. Dr. Robert A. Kehoe, an authority on the subject of the effects of prolonged exposure to air pollutants (see Chapter 87 of Air Pollution), believes that the investigation of causes (etiology) will have to proceed along different lines.3 He suggests that the harmful potentialities of contaminants can be defined by their physical and chemical composition, their concentrations, their ease of entry into the human body, and of absorption therein.
An unexpected factor that affects the etiology of long-range effects of air pollution is that new discoveries are made as scientific techniques are refined. Dr. Hans Neuberger's statement (in Air Pollution) that the use of the electron microscope in periodic air pollution surveys showed that particulate matter in the air was much higher than previously believed ("about 30 to 40 million particles per cubic centimeter") led Dr. Kehoe to comment on the skepticism with which previous standards must now be viewed.
In common with other investigators who cannot yet prove their hypotheses, Dr. Kehoe concludes with the observation that chronic disease of various types will probably occur "with increasing frequency and perhaps with increasing severity" in our general population as a direct result of atmospheric pollution.
TOLERANCE LEVELS
It is known that humans (and animals) can withstand certain amounts of air contaminants without harmful effects. However, long before air pollution was cause for government action, the public was concerned about illnesses and deaths caused by the contamination of air inside factories and workshops. Consequently, health officials and industrial hygienists are interested in finding the danger levels for substances emitted in industrial processes.
The American Conference of Governmental Industrial Hygienists has agreed upon the critical thresholds for a few substances. Termed "Maximum Allowable Concentration," they are based upon a long history and record of clinical study and experiments with animals.
Maximum Allowable Concentrations are expressed in parts per million by volume and in terms of exposure to one concentration of contaminant for eight consecutive hours a day, five days a week. Most of the standards are based on toxic effects but some are based on comfort or pleasantness.
For example, ozone has been given a threshold limit of 1 ppm. Headache and eye irritation occur around this level but serious injury to man from any concentration has yet to be reported. It is generally accepted that workers will not be harmed by daily eight-hour exposure to concentrations of sulfur dioxide up to 10 ppm. The highest concentrations of sulfur dioxide detected in the Los Angeles atmosphere during intense smog has been around 0.4 ppm. and the average about 0.1 ppm.
A complete list of industrial hygiene standards on Maximum Allowable Concentrations for gases, vapors, dusts, fumes, and mists is given in Table I, Chapter 5, of the Air Pollution Abatement Manual. Four related tables follow: Concentrations of Atmospheric Contaminants Reported in Various Locations; Odor Thresholds; Concentrations of Substances Causing Pain in the Eyes; and Exposures to Substances Causing Injury to Vegetation.
Some authorities believe that MAC standards may be appropriate for measuring tolerable limits of contaminants in the atmosphere or that they are the basis of a method of so doing. For example, E. M. Adams, author of Chapter 5, "Physiological Effects," of the Air Pollution Abatement Manual, observes that although MACs should not be applied per se to general air pollution problems, they will serve as guides in making "first approximations" of the health hazards of air pollution and that in some instances they may be appropriate.
Other groups feel strongly that MAC standards cannot be applied to air pollution. The California Department of Public Health, in its Clean Air for California, has summarized the reasons for the unsuitability of MAC standards to air pollution:
- The MACs are based on eight-hour exposure. We have insufficient data for knowing the duration of exposure to the several pollutants in the atmosphere.
- They do not take into account the physical state of the pollutants; for example, whether it is gaseous or not.
- They are based on limited age groups — industrial workers. In air pollution we are concerned with all age groups.
- They presuppose normal subjects. We are concerned with persons in varying states of health and disease.
- They do not take into account the possible effects of mixtures and interaction of the various pollutants.
Other sources also point out that the public is exposed 24 hours a day, seven days a week, rather than eight hours a day, five days a week. It has been suggested that the MAC for 24-hour periods should be one-tenth of the concentrations acceptable for an eight-hour exposure (Stern and Greenburg, "Air Pollution — The Status Today," American Journal of Public Health, January 1951). The workman has alternating periods of exposure and recovery, with recovery periods of about 16 hours. The reduced concentration would compensate for loss of recuperation time, although the one-tenth concentration is only a rule-of-thumb.
Point number 5 above (possible effects of mixing pollutants) has been emphasized by other investigators. The California Assembly Interim Committee on Air and Water Pollution points out that the MAC's that have been determined for industrial purposes are for the most part "based on the effect of a single material upon the worker's health, and little is known about the effects of combinations of two or three or perhaps 50 different contaminants in the atmosphere, such as may occur in a large city." Referring to the interaction of a number of contaminants, they say: "There is a question not only as to the simple additive effect of many pollutants, but also there is the unanswered question as to the possible synergistic effect of several contaminants, wherein the total effect may be far greater than the simple addition of each of the contaminants."
In spite of great difficulties in the way of determining maximum allowable concentrations for contaminants in the atmosphere of cities, investigators believe that such a goal should be pursued. Or at least that a great deal more should be found out about dangerous levels.
One of the technical obstacles to determining tolerance levels is the inability at the present time to measure accurately the amounts of toxic substances in the air. In fact, one source (Clean Air for California) says that "determination of the amounts in the air [of substances known to be toxic] is technically so difficult that considerable doubt exists as to the accuracy of quantitative information obtained with available methods."
In lieu of reliance on quantitative measures then, the first step is to detect the presence of toxic substances. Later tests may show that they are present in harmless amounts but the fact of their existence should be cause for suspicion and incentive for further investigation. For example, the following toxic substances have been found in the Los Angeles air:
LEAD. Lead, which is known to cause anemia, colic, and neurological disturbances has been found in appreciable quantities on many days throughout the year. It is one constituent for which no decrease had been achieved by control measures, at least up to 1951.
Recent observations indicate that lead is still present in considerably more than trace amounts, but its chemical form and health significance have yet to be evaluated.
ACIDS. During periods of intense smog, relatively high concentrations of mineral acids sometimes occur. Sulfuric acid, a major component of the mineral acids present, induces the effects of sulfur dioxide.
SULFUR DIOXIDE. Sulfur dioxide, one of the most frequent pollutants of industrial cities, is a colorless irritant gas. It causes intense irritation of the mucous membranes of the eyes and nose, and may also affect the mouth, trachea and bronchi.
Six to twelve ppm. is said to be the minimum concentration necessary for eye irritation. The maximum level of sulfur dioxide reported in Los Angeles in recent years has been less than 1 ppm. One can find no evidence of injury to man even by long-standing exposure to such low concentrations.
OXIDES OF NITROGEN. These are irritant gases capable of producing injury to the lungs without much warning. Effects of poisoning include yellow sputum, chocolate brown blood, shortness of breath and death from asphyxia (oxygen lack).
The minimum concentration known to cause irritation of the eyes and nasal passages is about 75 ppm. Over 100 ppm. is considered dangerous for a short exposure of one-half to one hour. Highest concentrations found in the Los Angeles area have been less than 2 ppm.
ORGANIC SUBSTANCES. For the myriad of organic substances which as air pollutants might induce acute or chronic irritation, no tolerance limits have been established. Organic substances with toxic potentialities found in the air of Los Angeles in more than trace quantities include hydrocarbons of various types (chlorinated hydrocarbons: aliphatic hydrocarbons, saturated and unsaturated, and their peroxides and ozonides; aromatic hydrocarbons, especially the polycyclic carcinogenic substances such as benzpyrenes) aldehydes, carbon monoxide and acids.
HYDROCARBONS. Total hydrocarbons (saturated and unsaturated) have been found in amounts of 1–2 ppm. Tests have shown chlorinated hydrocarbons in concentrations of 0.1 ppm.
Benzpyrene, a well-known cancer-producing hydrocarbon found in the air of English cities, has been detected in the air of Los Angeles, as already noted. Whether Los Angeles air now contains benzpyrene or other cancer-producing substance in sufficient quantity to produce cancer is not known. Hydrocarbons from Los Angeles air when concentrated and applied to the skin of animals can cause tumor formation. Thus far no lung cancer has been produced in experimental animals by inhalation of these substances. Because pollution of Los Angeles air with hydrocarbons is recent — so far as we know — and because the development of cancer sometimes takes 20 to 30 years, the significance of hydrocarbons may not be determined for decades.
ALDEHYDES. Acrylic aldehyde (acrolein) came into use as a tear gas in World War I. One ppm causes an irritation of the eyes and nose which becomes intolerable in five minutes. This substance has been identified in Los Angeles air, but in amounts insufficient for measurement.
CARBON MONOXIDE. Carbon monoxide causes more fatalities in peacetime than any other asphyxiant gas Since it is colorless, odorless, tasteless and nonirritating, it acts suddenly without warning. It combines with the hemoglobin of the blood, thus preventing the transportation of oxygen to the tissues. Depending on severity of asphyxiation, acute effects may range from a mild frontal headache to dizziness, nausea, severe throbbing headache, weakness, loss of consciousness, respiratory collapse and death.
Measurements of Los Angeles air have shown concentrations as high as 40 ppm. It is estimated that a concentration of 200 ppm. for 2 hours is necessary to produce minimal effects in humans. Authorities disagree as to whether prolonged exposure to low concentrations produces definite pathologic change.
A TOXICOLOGIC PARADOX. Certain substances in extremely low concentrations can cause uncomfortable (but not serious) effects while higher concentrations of the same substances do not increase the effects. For example, as little as 1 ppm. of iso-amyl acetate can cause irritation of the respiratory tract, headache, vertigo, fullness of the head, drowsiness, oppression in the chest, and nausea — a picture resembling the "smog syndrome." Yet an amount many thousand times greater does not produce death or even unconsciousness in animals. (The substances in Los Angeles air may not act like this — but it is noteworthy that at least one substance behaves in this manner.)
INTERACTION EFFECTS — PHYSICAL AND CHEMICAL. The complexity of the problem of determining tolerance limits is compounded by the mixing and chemical interaction of numerous pollutants, aided by such agents as heat, light, and inorganic substances. For example, the naturally occurring oxygen of the air may interact with oxides of nitrogen by means of the energy from the sun to produce new substances; these in turn may react with hydrocarbons from various sources to produce substances with eye-irritating properties. Thus, irritant properties may arise not from a single pollutant, but from the interaction of naturally occurring gases and unburned emissions from various sources.
The possibility cannot be overlooked that production of irritants is aided by catalysts, chemical accelerators discharged into the air from industry. The petroleum industry alone uses dozens of catalysts in its various operations. Many of these when liberated could conceivably accelerate the reactions among air pollutants to create new and unusual substances.
PHYSIOLOGIC EFFECTS — DEPOSITION AND RETENTION OF POLLUTANTS. The adsorption of gaseous material onto minute particles of matter may play a role in the airborne characteristics of a substance, or in its capacity to penetrate the respiratory tract. Some gaseous substances, ordinarily absorbed in the nose and upper respiratory passages, may attach themselves to tiny particles of solid or liquid matter and thus descend into the delicate lung tissues.
As much as 10 times greater amounts may be deposited in the lungs when the particle size diminishes to one-fifth. The depth and rate of respiration may also affect particle deposition in the lung. Increased rate and depth of breathing as in exercise and hard physical labor will greatly increase the amount deposited. The amount of air inhaled at hard labor may be as much as 14 to 16 times that at rest. Concomitant changes in pulse rate and circulation cause more rapid dissemination of pollutants throughout the body thus tending to increase toxicity.
(Source: Clean Air for California.)
The Automobile in Air Pollution
How significantly exhausts from motor vehicles contribute to air pollution is still controversial.4 It is generally agreed, though, that the unburned hydrocarbons present in motor exhaust fumes constitute a major component of contaminated air. An indication of the enlarging factor of motor vehicles in the air pollution picture is seen in the following figures on motor vehicle registrations (Source: Statistical Abstract and Bureau of Public Roads, U.S. Department of Commerce).
| 1950 | 1955 (Estimated) | |
|---|---|---|
| Automobiles | 40,185,000 | 50,954,000 |
| Trucks and Buses | 8,382,000 | 10,347,000 |
| TOTAL | 48,567,000 | 61,301,000 |
Motor fuel consumption in the United States has been estimated to be about 150 million tons a year. It has been calculated that one gallon of gasoline produces 1,000 cubic feet of exhaust gas on a dry basis.
Motor exhaust fumes contaminate the atmosphere in two different ways: pervasively (diffused throughout the atmosphere over a wide area) and locally.
The pervasive contribution of motor exhaust fumes to air pollution was discovered in Southern California, where per capita car ownership is higher than any other place in the world. According to Clean Air for California motor vehicles now consume more than 4 million gallons daily. But:
Vehicles do not burn all of this. Actually about 1,200 tons of gasoline vapors (hydrocarbons) are released from motor vehicles each day. The bulk of this comes from automobile exhausts but some escapes through the breathing of automobile tanks and carburetors. Motor vehicles emit significant quantities of nitrogen oxides, and relatively smaller amounts of aerosols, sulfur oxides, aldehydes, ammonia, as well as organic acids and other organic substances, Other pollutants result from the use of additives to gasoline such as tetraethyl and detergents. The use of motor oils and their additives are another source of pollution.
To determine the amount of foreign material — chiefly hydrocarbon emissions — introduced into the atmosphere by automobiles in Los Angeles County, Stanford Research Institute investigated a number of factors. Results of this investigation are published in The Smog Problem in Los Angeles County, January 1954, distributed by the Western and Gas Association, 10 West Sixth Street, Los Angeles 14, from whom copies can be obtained.
One of the most significant findings was that the amount of hydrocarbon emission is much higher under the driving conditions of deceleration and idling than under acceleration and steady driving. Type of gasoline burned made no difference in the exhaust composition but age of automobile did under deceleration and idling conditions. However, regardless of age of automobile, the greatest amount of hydrocarbon emission occurred under deceleration and idling.
Similar findings were made in a General Motors experiment involving 150 automobiles. The machines were placed on idling and later on deceleration. Tests of the exhausts on the idling cars disclosed that from 1 to 28 percent of the gasoline used was discharged unburned into the air. Under decelerating conditions, the unburned fuel loss ranged up to as much as 60 percent. (Source: Los Angeles Times, January 25, 1955.)
Several proposals for the reduction of hydrocarbon emission from automobile exhausts have been made. However, no inexpensive and practicable method has yet been found. The automotive industry has developed a device that will cut the release of hydrocarbons from exhaust as much as 30 to 50 per cent by automatically cutting off waste during deceleration. However, industry spokesmen say that it will be two or three years before the new apparatus can be mass-produced and that it will probably cost about $25. According to Clean Air for California, two other approaches to the problem are now being studied: the inclusion of catalytic converters and afterburners and the redesign of the engine for more efficient combustion.
Private vehicles are not the only offenders; gasoline powered buses have recently been scrutinized for their part in air pollution. In New York City, the Board of Air Pollution Control has adopted three rules regarding their operation: all gasoline buses shall be equipped with fume-reducing devices; bus drivers are required to shut off their engines within three minutes after arrival at terminal points; any bus that is found to produce excessive objectionable fumes shall be removed from service for repairs. Gasoline buses compose only about one-fifth of all the buses in New York City.
Diesel buses are coming to the attention of air pollution officials in some cities in response to numerous complaints of smoke and fumes. Contrary to gasoline engine operation, the diesel engine vehicle emits more fumes when accelerating.
Upon instruction of the Detroit common council, the Smoke Abatement Bureau of that city submitted a report on the emission of odor, smoke, gas, etc., from diesel engine coaches. The following points are taken from this report, dated June 20, 1955.
The Diesel engine emits only about one-one-hundredth as much carbon monoxide as the gasoline engine.
The Diesel engine emits no lead compounds.
The Diesel engine emits 40% more oxides of nitrogen than the gasoline engine. This is especially noticeable when pulling away from a stop.
The Diesel engine emits 40% more formaldehyde than the gasoline engine, except when decelerating.
The Diesel engine emits 60% less hydrocarbons than the gasoline engine. This is especially noticeable when decelerating when the Diesel emits one-sixth the amount of hydrocarbons compared with the gasoline engine.
The biggest complaint against the diesel bus is the odor of the exhaust, which apparently is due to the higher formaldehyde content.
The Smoke Abatement Bureau believes that the problem of visible smoke from diesel buses is one of engine maintenance and fuel specification. Under these conditions, there is no advantage or disadvantage between diesel buses and gasoline buses with respect to visible smoke. The problem of odor is less easily resolved, though engine maintenance and fuel specification will reduce it somewhat. However, even with proper maintenance, there is a distinctive diesel odor.
This report also states that the automotive industry is engaged in a research development program to reduce undesirable engine exhaust gases. Now, all motor vehicles emit characteristic odorous gases. "Large vehicles, such as buses, are more disturbing than small vehicles because the size of the 'blast' is larger." Fumes from buses thus affect the atmosphere both locally and generally, though their share in smog in the Los Angeles area is considered to be minor.
Propane is being studied as a fuel for buses but apparently information about it is still inconclusive. A recent report made by the Detroit Smoke Abatement Bureau remarks that:
Propane powered buses have been reported to be freer of smoke and odor problems than Diesel and gasoline buses. We have not verified this by observation of Bureau personnel. The limited technical literature indicates that there is less formaldehyde from propane buses. With respect to the other gases, they produce no lead compounds, and are reported to stand in between gasoline and Diesel buses with respect to the emission of carbon monoxide, oxides of nitrogen and hydrocarbons.
In area-wide air pollution, carbon monoxide apparently is not a serious factor.5 However, there are signs that it may have unhealthy effects on a local scale. Field tests are being made in London to determine whether carbon monoxide gas from vehicles is reaching concentrations in the busier streets powerful enough to cause headaches and other symptoms of carbon monoxide poisoning among persons exposed to fumes in their daily employment, such as policemen and street cleaners.
Smog
Although the word "smog" derives from a combination of the words "smoke" and "fog," it now includes types of atmospheric pollution where smoke is not a factor. In Southern California — where almost no coal is burned — it refers to the characteristic condition of polluted air in that area. While recognizing that the word "smog" can refer to different kinds of air pollution in different places, here we limit its use to the kind of air pollution prevalent in the Los Angeles basin.
An aggravating factor in Southern California smog is a combination of topographic and meteorological conditions so unique that many observers have thought that the smog, too, was unique. Now, however, it is recognized that area-wide pollution of Southern California can occur without being accompanied by the peculiar physical features of that area.
At the present time it seems unlikely that smog occurring elsewhere will ever reach the menacing proportions that it has in Southern California. However, observers believe it is significant that smog has occurred elsewhere and they warn that the manmade pollutants responsible for smog in Los Angeles are on the increase in other urban areas. Furthermore, temperature inversions are more widely occurring than had been believed.
Therefore, the general principles of the Los Angeles smog situation are applicable to other urban areas. Government agencies and industry, alike, can profit from issues brought to focus by an extreme example.
The characteristic effects of smog are eye, nose, and throat irritation; reduced visibility; and damage to vegetation. When we come to the matter of what smog is, how it is formed, and what its principal sources are,we enter a field of controversy and some mystery. In spite of considerable research, there are still unknowns. Although positive statements can be found in news stories and other places, scientific and medical articles are more cautious. Phrases such as "may cause," "are believed to include," "may be responsible," are frequent.
Some factors are known: an extensive inversion layer (a layer of warm air above a layer of cold air) exists that acts as a lid over the basin formed by mountains on three sides; there are gentle winds that often are not strong enough to break up an inversion layer, which may extend for 400 miles along the ocean front and from the coast west to the Hawaiian Islands; there is brilliant sunshine capable of energizing a variety of chemical reactions. These natural conditions, everyone agrees, are implicated in smog.
Discharges of manmade pollutants into an atmosphere modified by these natural conditions create smog. The composition of smog is complex and it is not certain that all of its components or even all of its significant components have been identified. However, agreement with the theory of smog formation developed by A.J. Haagen-Smit of the California Institute of Technology is widespread.
According to his theory, invisible hydrocarbon gases are transformed by the action of sunlight and ozone in the atmosphere into a series of irritant substances known as aldehydes. Formaldehyde is one of the series. But this is not by any means the whole story. One of the big mysteries is ozone: where does it come from in such quantities that large-scale reactions take place between it and other substances?
One of the fascinating observations on smog is that high ozone concentrations occur with periods of severe smog: the denser the smog the higher the ozone count.6 Average ozone levels in most areas of the world where ozone measurements have been made are very low, ranging from one to six parts per hundred million (.01–.06 ppm.). The only exceptions to this are Fairbanks (Alaska), the Mojave Desert (California), and Detroit, where between 20 and 30 parts per hundred million (.2–.3 ppm.) have been measured. Subsidence from the upper atmosphere has been postulated to explain these high readings. In Los Angeles, on the other hand, readings of 30 to 40 parts per hundred million are common during smog periods and values up to 90 parts per hundred million have been measured. (It should be remembered that 1 ppm. is the MAC value.)
The explanation for ozone formation in Los Angeles atmosphere appears to be photochemical reactions involving sunlight and materials in the atmosphere. Again, this explanation seems to be widely accepted. Uncertainty still exists, however, as to just, what these materials are.
The Stanford Research Institute, after a series of experiments, concluded that "...it has been established that ozone is still produced when nitrogen dioxide is removed and when aldehydes and ketones are removed." However, in an article in the July 1955 Scientific Monthly, "Some Scientific Aspects of the Urban Air Pollution Problem," by Lauren B. Hitchcock and Helen G. Marcus, it is stated that "there is mounting evidence" that organic compounds react in the presence of sunlight and nitrogen oxides "to produce oxidized organic compounds and ozone." And later in the same report of the Stanford Research Institute (The Smog Problem in Los Angeles County) the viewpoint implied above is modified:
It thus appears to be established that the bulk of the ozone in Los Angeles is formed by a photochemical mechanism not requiring the presence of nitrogen dioxide. However, the photochemically active material may be a compound formed previously by a reaction involving nitrogen dioxide.
Haagen-Smith's experiments also show that hydrocarbons react photochemically with nitrogen dioxide to form an oxidant that presumably is ozone. The aldehydes apparently are among the intermediate or secondary products of this reaction. This reaction is only one of several that are believed to take place but judging from the literature on the subject, it is perhaps the most important as far as irritation to human tissue is concerned.
Aerosols seem to be chiefly responsible for reduced visibility when there is no natural fog. The term aerosol is used to designate particles dispersed in a gaseous medium. It was coined by analogy to colloidal dispersions in a liquid medium, which are called sols. Aerosols produced artificially are used to spread material very thin and still obtain covering power. It is suspected that by virtue of synergistic action between toxic gases and aerosols, gases are absorbed on particles and carried into the lungs.
Aerosols are both liquid and solid but those involved in visibility reduction are mainly liquid droplets ranging in diameter from 1/10,000 to 1/100,000 of an inch. "These droplets," according to Stanford Research Institute, "contain water, dissolved organic and inorganic material, and appear to be surrounded by a tough 'skin' or envelope. They are remarkably persistent in air, and will exist for considerable periods of time at relative humidities below 30 per cent."
Crop damage, another harmful effect of smog, takes the form of silvering, bronzing, and necrosis of the lower leaf surfaces. The oxidants formed by the reaction of hydrocarbons, ozone, and oxides of nitrogen, in sunlight, are again believed to be the toxicants causing these effects. The principal economic crops affected are alfalfa, beets, beans, celery, spinach, romaine lettuce, and oats. Damage has been estimated to be more than one million dollars annually.
We arrive now at the most controversial of all aspects of the smog problem: where do the air pollutants come from and who is responsible? One thing can be said with assurance: there is no single source. Throughout the history of smog in Southern California there has been a tendency to single out on an emotional basis, one kind of a process and say "this causes smog." For several years, the petroleum refineries were held sole agents. For a time, backyard incinerators were said to be the cause. At present, the automobile is the recipient of most of the blame.
Needless to say, it is fallacious to conclude that one factor — no matter how major a role it may play — causes a type of air pollution as complex as smog. Possibly this kind of reasoning is a carryover from the fruitful analysis of smoke that led to an essentially single source — the burning of coal. Nevertheless, much progress has been made in identification of the major contributors to smog.
From Clean Air for California:
Where does it come from?
URBAN AREAS
More than 40 foreign substances have been identified in the atmosphere of California coastal cities. The principal sources of some of these substances are identifiable. For example, hydrocarbons in the atmosphere arise from motor vehicle exhausts, petroleum refinery processes, the storage and marketing of petroleum products and from other sources. Nitrogen oxides occur in exhausts from automobiles, household and industrial fuel, and oil and gas burners. Smoke may be traced to domestic incinerators, open fires, industrial and municipal incinerators and fuel oil burners. Dusts and fumes result from certain mineral and earth processes, metals industries, grain and feed processes and a wide variety of chemical industries. Sulfur oxides are emitted from fuel oil burners, petroleum processes and chemical processes.
Several organizations have made estimates of the kinds and amounts of air pollution originating from urban areas in California, particularly Los Angeles. The following appear to be major contributors to air pollution in Los Angeles, and other California metropolitan areas:
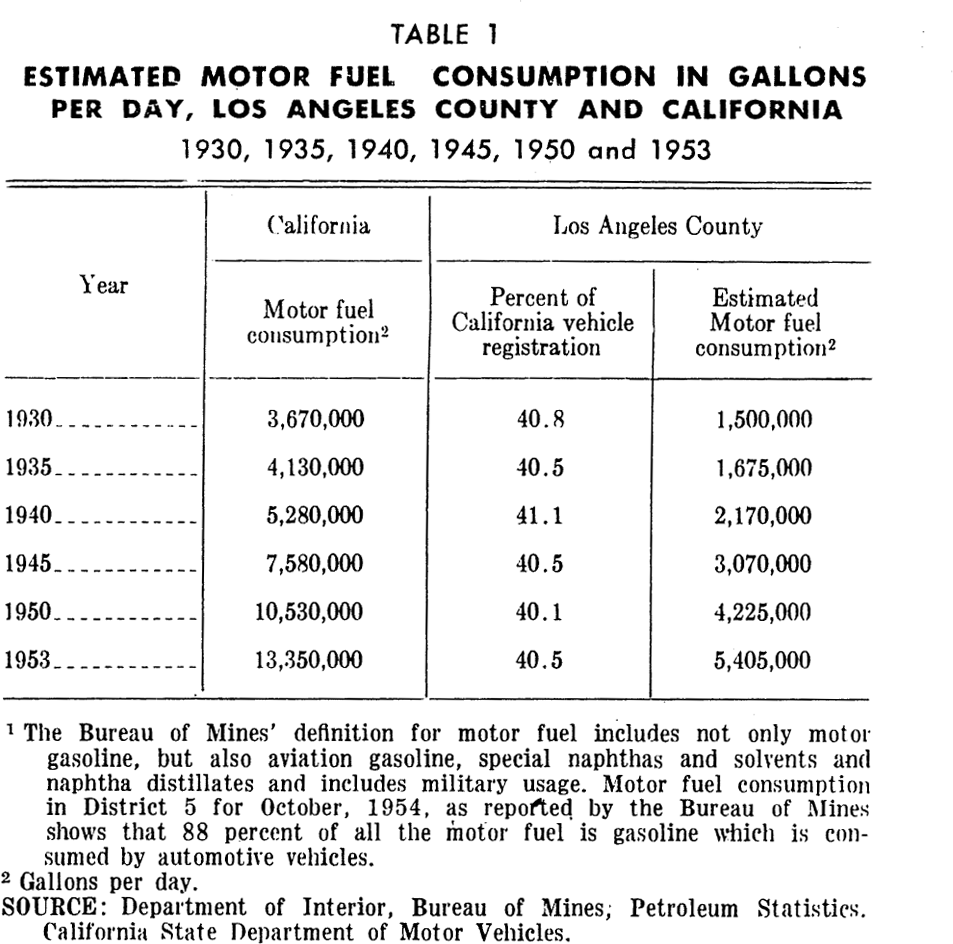
Motor vehicles: Southern California has become the land of automobile travel. The number of vehicles registered in Los Angeles County increased from 871,773 in 1930 to 1,229,194 in 1940. By 1953 the number had become 2,427,566 which represents 412 motor vehicles per 1,000 persons.
The increase in number of automobiles is reflected in an increase of motor fuel consumption which is shown in Table 1 (see appendix for tables) for Los Angeles County from 1930–1953. Motor vehicles in Los Angeles County now consume over 4,000,000 gallons of gasoline daily. Vehicles do not burn all of this. Actually about 1,200 tons of gasoline vapors (hydrocarbons) are released from motor vehicles each day. The bulk of this comes from automobile exhausts but some escapes through the breathing of automobile tanks and carburetors. Motor vehicles emit significant quantities of nitrogen oxides, and relatively smaller amounts of aerosols, sulfur oxides, aldehydes, ammonia, as well as organic acids and other organic substances. Other pollutants result from the use of additives to gasoline such as tetraethyl and detergents. The use of motor oils and their additives are another source of pollution.
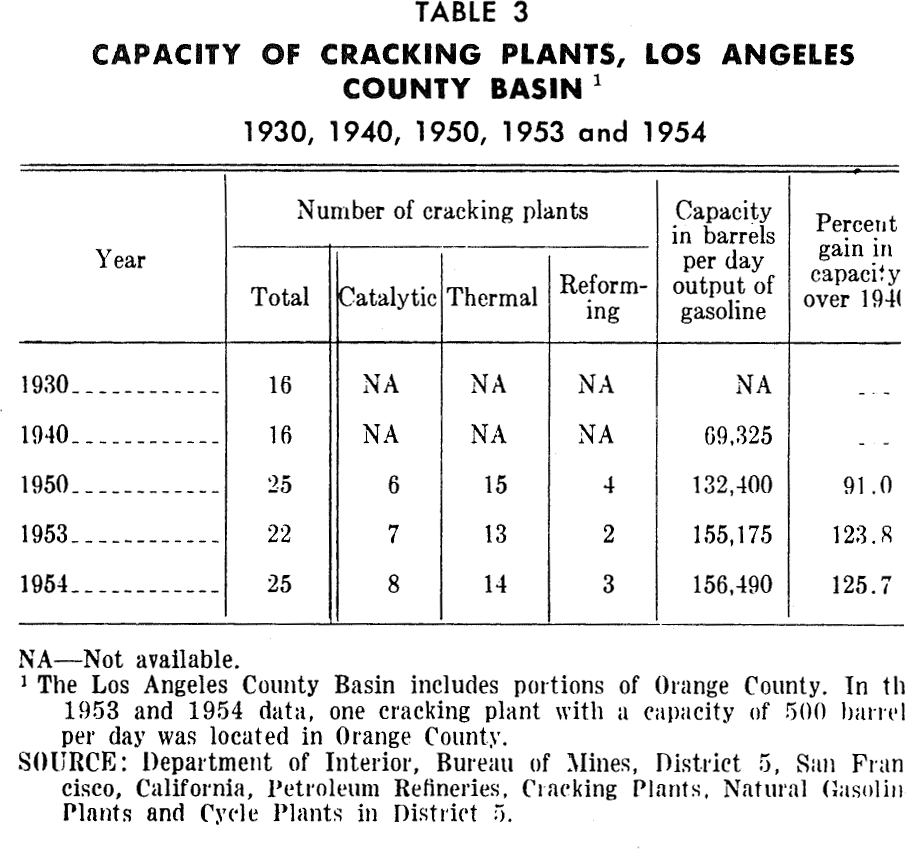
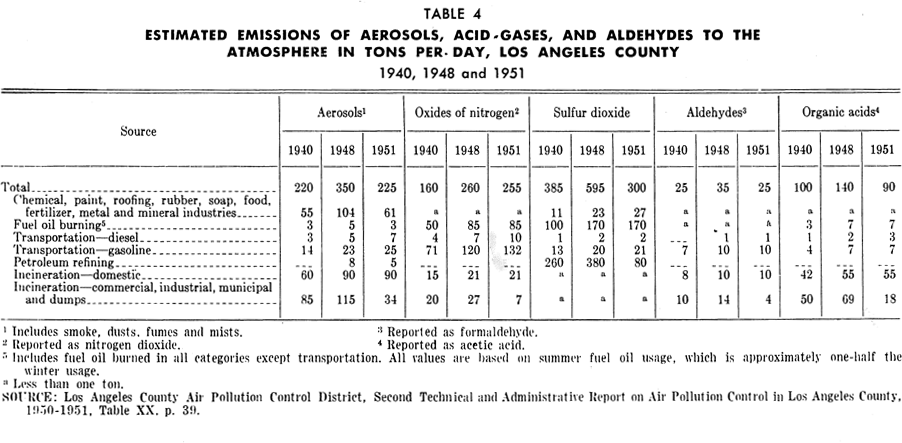
Oil refineries: Oil refineries have greatly increased their capacity in the Los Angeles basin during recent years. Tables 2 and 3 illustrate the expansion in capacity of "straight-run" refineries and gasoline "cracking plants" in the Los Angeles basin from 1930 to 1954. Gasoline cracking plant capacity has increased over 125 per cent from 1940. Petroleum refining gives rise to substantial amounts of hydrocarbons in the air. It also emits sulfur oxides, nitrogen oxides, aldehydes, acids, and organic substances. In Los Angeles County, emission of sulfur oxides from refineries is said to have been reduced from 380 tons per day in 1948 to 80 tons per day in 1951 (Table 4).
A 1951 estimate indicated that refineries discharged into the air 830 tons of hydrocarbons per day. The estimated emissions as of January, 1955, show a reduction to 210 tons per day of hydrocarbons. Control measures instituted during the four-year interval account for this reduction in spite of expansion in petroleum production.
Fuel oil and fuel gas: For indoor heating in Los Angeles County, as elsewhere in California, fuel oil and fuel gas are used almost exclusively. Fuel gas consumption increased from approximately 100,000 million cubic feet in 1930 to 120,000 million cubic feet in 1940, and to 340,000 million cubic feet in 1949. Consumption of fuel oil for heating expanded from 1,000,000 barrels in 1934 to 1,900,000 barrels in 1940, and to 4,900,000 in 1950.
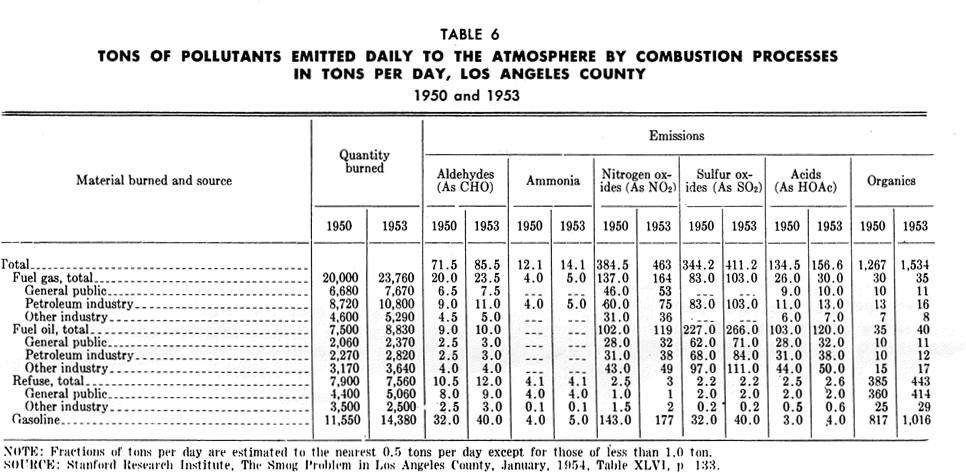
The fuel gas consumed by the general public and industry, other than the petroleum industry, is natural gas which burns with little smoke. Table 6 shows the amounts of various emissions from the burning of natural gas; these emissions include sizable quantities of nitrogen oxides, smaller amounts of aldehydes, as well as organic acids and other organic substances. The petroleum industry consumes byproduct fuel gas from its own operation. Emissions from combustion of this gas are considerably greater than from natural gas.
From fuel oil burning, large quantities of nitrogen oxides and sulfur oxides are emitted, as well as a number of other substances.
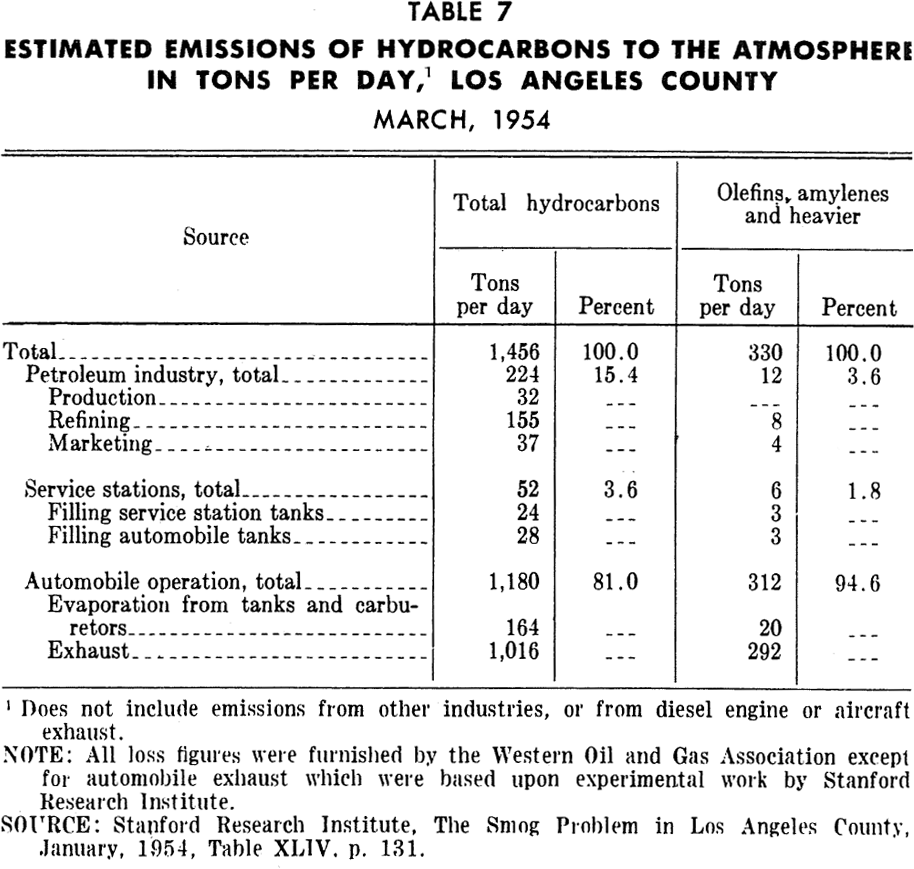
Gasoline marketing and distribution: Discharges of hydrocarbons, as shown in Tables 5 and 7, to the atmosphere occur at many points in the system for distribution of gasoline. These points include trucks, railroad cars, seagoing vessels, bulk gasoline terminals, retail station tanks and vehicle tanks. Losses occur from filling of tanks as well as breathing during storage. However, the amounts lost are apparently much smaller than from either motor vehicle use or petroleum refining.
Refuse incineration and disposal: An unusual system of refuse disposal has grown up through the years in Los Angeles County. Refuse is usually separated at the point of origin into garbage, combustible rubbish and noncombustible rubbish. The garbage is most often used for hog feed and the noncombustible rubbish usually disposed of by land-fill methods. The combustible rubbish is burned by household, municipal or industrial incineration.
The relative importance of refuse burning in smog formation is still undetermined. A wide variety of substances including both gaseous and particulate matter arises in sizable amounts from incineration.
In 1955 some 1,500,000 backyard incinerators burned an average of 6,000 tons of refuse per day in Los Angeles County. Four municipal incinerators located in the Los Angeles County area burned 400 tons of refuse per day in 1951. Industrial wood-burners consumed 1,500 tons of material, and other commercial and industrial incineration accounted for an additional 1,000 tons of material. Tables 4 and 6 indicate the amount of emissions from refuse incineration.
Manufacturing, processing and chemical industries: Industrial activity in urban areas contributes an appreciable total of pollutants to the air. Each manufacturing or processing industry gives rise to specific waste substances; thus the variety and quantity of their airborn discharges are dependent on the local industrial pattern. Tables 4 and 5 include some illustrative data as to emissions from the chemical, paint, roofing, rubber and other industries.
Exotic sources: Air pollution may arise outside the local area and be carried into it by air movements. Radioactive materials are carried into the California atmosphere from atomic-bomb tests and other operations involving radioactivity. Dust and particulate matter may come from forest fires, wind actions and other natural occurrences. Pollens which cause hay fever may be transported for long distances. The total amount of pollutants arising outside the immediate urban areas is often substantial.
Miscellaneous sources: Many other sources contribute to air pollution in California cities, including:
1. Combustion of fuels other than petroleum products and gas.
2. Locomotive and aircraft fuel combustion
3. Material from the abrasion of rubber tires
As an illustration of the complexity of the origin of a single atmospheric pollutant the case of lead may be cited. It comes from auto exhausts and from many industrial sources including lead refining, nonferrous foundries, gray iron foundries, and metal dye casting.
Although it appears that the major sources of pollution in California urban communities have been recognized, until the air pollution problem is solved no source within a city should be finally dismissed as unimportant.


Other Aspects of Air Pollution Control
Because of space limitations, we have not been able to report on certain other aspects of progress in air pollution control over the past five years. Among those that are meritorious are the efforts of industry to develop processes and equipment that will reduce or eliminate emission of pollutants at the source and industry's cooperation with government agencies. This phase of air pollution control is, in itself, a long story. The railroads, the steel industry, the chemical industry, the petroleum industry, and more recently the automobile industry have made outstanding contributions. Nevertheless, it must be admitted that smoke has been successfully abated only after the application of government controls. We have no reason to believe that air pollution will be abated without laws and their enforcement.
This brings us to the question of legislation, which, too, is a long story. The governmental level of regulation has been the subject of much discussion. Though somewhat clarified by the recent federal law declaring that the control of air pollution is the primary responsibility of state and local governments and authorizing federal funds for air pollution research, the question is still open on what constitutes "local government." With the exception of a few counties, local has meant municipal — "generally to industry's great benefit," comments a recent Fortune article.
There is, apparently, a growing belief that the smallest unit of government control should, at the least, coincide with the urban area affected by pollution. A regional approach has been recommended. Pittsburgh's program was ineffective until Allegheny County adopted comparable legislation. At the present time, Detroit is concerned about out-of-town sources of air pollution (especially the large steel mills, chemical plants, and foundries, which discharge materials that combine to form aerosols and gases not unlike those composing smog and that blow across municipal boundaries).
Another important aspect only touched upon here is the problem of refuse disposal. Although backyard incinerators are peculiar to Los Angeles (except, perhaps, for their use in much smaller cities), what to do with rubbish and garbage is plaguing more and more cities. In New Jersey, fumes and smoke from burning refuse dumps have caused low visibility on the freeways and have lowered ceilings at airports. Some of the references at the end of this report contain more information on this problem, which, it is submitted, is essentially one of municipal housekeeping.
Also not reported here are the many developments in recording and measuring devices, the technique of aerometric surveys, and advances in contaminant-removal equipment. A great deal of information is available, much of it technical.
Finally we come to the omission of information on the air pollution problems and programs in other metropolitan areas. Space again is the limiting factor. However, it is also believed that a report on the two classical types of air pollution — smoke and gaseous compounds — is more informative than a description of what is happening in nearly every metropolitan area.
To document the observation made earlier that there are signs of smog and gaseous air pollution (as contrasted with smoke pollution) in a number of other places, mention is made of the following cities from which reports on air pollution problems have been made: Elizabeth, New Jersey, Detroit, New Orleans, Ottawa, Canada, Seattle, Portland, Oregon, and the District of Columbia. Smog damage to plants has been observed in the San Francisco Bay area (and other California cities), New York, Baltimore, Philadelphia, London, Manchester, Copenhagen, Paris, Algiers, Saõ Paulo, and Bogota.
Planning Considerations
It is hard to make a thoroughly realistic appraisal of the importance of clean air but perhaps a world-wide view will put it in perspective.
During the International Geophysical Year starting July 1957, scientists will study the theory that contamination of the earth's atmosphere is having far-reaching effects on weather. One industrial engineer (Dr. John G. Hutton of General Electric) believes that man is contaminating the earth's atmosphere faster than nature can clean it (Source: Arkansas Democrat, September 23, 1955). The result is a greenhouse effect, created by a belt of carbon dioxide encircling the earth. He hypothesizes that recent erratic weather phenomena — such as extreme temperatures and hurricane behavior — may be explained by this effect.
Another source (the 1954 annual report of the California Institute of Technology) discloses that burning of huge quantities of coal and petroleum since 1840 has enriched the earth's atmosphere in carbon dioxide and radioactive carbon. (Source: Los Angeles Times, November 26, 1954). The present carbon dioxide content of the atmosphere is about 5 per cent greater than it was 100 years ago. According to the report, "If this trend continues it is conceivable that addition of carbon dioxide to the air eventually may affect aspects of our natural environment. This might include changes in weather as well as in plant and animal growth."
It is more and more apparent that air pollution is not a minor problem and that people are worried about it. The course that the smog problem in California has taken is viewed as a pattern of what may happen in other cities.
Out of necessity, the situation there may possibly be brought under control before similar manifestations develop in other cities to the point where action is demanded by the public. That is, it is possible that the automobile industry will succeed in building into cars a device that substantially reduces emission of hydrocarbon vapors from exhausts and that the petroleum industry will similarly cut down on the release of toxicants into the atmosphere. If these two goals are achieved, then other localities will benefit greatly.
However, the lessons to be learned from smog in California go beyond identification and control of two major sources of air pollution. One of the chief phenomena in this situation — and one that is somewhat obscured by its more conspicuous manifestations — is the tremendous increase in population and industry that has occurred there in the last ten years.
We know that the same thing is happening — at a slower but none the less relentless pace — in other parts of the United States and in Canada. Consequently, the tendency will be toward the emission of (1) more kinds of pollutants, (2) greater quantities of pollutants, and (3) potential interactions of unknown composition and toxicity.
If we assume that the automobile industry fails to develop and market a device to reduce exhaust hydrocarbons, we can predict that automobile fumes will continue to contaminate the atmosphere at an increasing rate with an increase in car ownership. If, on the other hand, such a device is perfected and widely applied, then we can predict that air pollution from this source will be greatly reduced. However, it should be noted that total emissions from exhausts will increase over a period of time with an increase in the number of vehicles. How significantly this source will contribute to future air pollution will depend on the effectiveness of the device and the number of vehicles.
Another source of potential increases in the chemical industry, which is on the eve of expansion to unprecedented magnitudes. Some measure of this expansion is seen in the recent report by the Department of Health, Education and Welfare to a Senate appropriations committee predicting that by 1975 production of synthetics involving chemicals will be 30 times as great as it was in 1950. One of the reasons for this expansion is that of all industrial processes, the chemical industry (and petroleum refining) lends itself most conveniently to automatization. Here, too, the rising number of plants will increase the total amount of contaminants over a period of time, even though that total may be initially reduced far below present levels.
In addition to population, industry., and automobiles, the other gross variables in smog formation are known to be weather, wind, and terrain. In any given locality, these factors can be determined with some accuracy and it is here that the city planning agency can probably make its most significant contribution to air pollution control: the special function of planning in government is prediction.
A planning agency can gather information on air pollution potential from industrial land use surveys, from economic base studies, and from traffic surveys and projections. Less directly, but just as inevitably, population predictions will figure in estimates of future air pollution. A planning commission should inform itself on new industrial processes and how they may affect the composition of the atmosphere.
In their advisory capacity, planning commissions are concerned about the location of future industries. To be realistic, it must be pointed out that in a situation already severe — such as Los Angeles County — judicious downwind location of new refineries is not going to make much difference. On the other hand, many smaller communities are still able to regulate the location of new industries and it is in these localities that consideration of wind patterns will be helpful. However, we know from experience that it does no good to rake up our own leaves if our neighbor doesn't bother and what is downwind from one city may be upwind from another.
Density controls have been suggested as a means of diluting the effects of noxious or toxic effluents. However, it is somewhat questionable if this measure would make any difference to the total volume of emanations resulting from a large number of really big contaminators.
Since the application of performance standards to the effects of industry was the subject of two previous Information Reports (Nos. 32 and 78) it is only pointed out here that such regulations are based on control or elimination at the source. It is becoming increasingly evident that the only effective road to clean air is to prevent the air from getting dirty in the first place.
Conclusions
Successful smoke abatement programs show that the goal of clean air must exist before abatement will be accomplished. It apparently is not enough to say "something must be done about air pollution" or even "it must be stopped." The community at large must want clean air — a positive goal. And the community includes business and industry, the automobile-driving public, and government.
An important administrative part of a clean-air program is a goal of overall total reduction of atmospheric contamination. This goal should be realistic: it should be one that will achieve clean air and it should be one that can be accomplished. Since unknowns are still a part of the air pollution picture, the means of overall reduction will have to be within the limits of known techniques. Therefore, the goal might be defined in terms of stages and time: a percentage reduction within the first year, a higher percentage within the second year, etc.
Where many different economic groups are involved in an air pollution problem, interests will conflict. Probably, therefore, the evidence on what sources cause air pollution will also conflict. There will be claims and counterclaims. Therefore, some skepticism should be maintained and reports should be examined critically.
Essential to a successful air pollution abatement program is the rule of "no exceptions." This rule has been tested in St. Louis and Pittsburgh-Allegheny County. It is an intrinsic part of the British Clean Air Bill. The doctrine of exceptions for "hardship cases" is dangerous when applied to contaminating sources because of the cumulative nature of air pollution.
Experience and feasibility examination of other methods leads to the conclusion that the most effective method of reducing air pollution is through elimination at the source.
Endnotes
1. The current report does not supersede Information Report No. 20, nor does it duplicate any of its information.
2. New York City has been in the news recently with reports that the more than 30 million tons of soft and hard coal and various grades of fuel oil burned each year produce more than 1–1/2 million tons of sulfur dioxide gas, which in turn produces more than 2 million tons of sulfuric acid. Compare this with the approximate 150,000 tons of sulfur dioxide produced annually in the Los Angeles area.
3. One of the traditional methods of proving a causal relationship between pollution and injury is by establishing the following four points: (1) a toxic effect has occurred; (2) a pollutant capable of causing this effect has been present; (3) there has been an excessive exposure to the pollutant; (4) other causes of the toxic effect do not exist. (See Chapter 5 of Air Pollution Abatement Manual, published by Manufacturing Chemists Association.)
4. This statement should be modified in reference to Southern California, where it has been fairly well established that exhaust fumes from motor vehicles — chiefly passenger cars — are a major contributor to smog.
5. As the result of more efficient engine performance, carbon monoxide emission from automobiles has decreased considerably in the United States. Carbon monoxide values in Los Angeles atmosphere recently were measured at 33 ppm. The average value of 88 ppm. was reported by the U.S. Public Health Service in 1928. Maximum Allowable Concentration value is 100 ppm.
6. This fact has been utilized in the Los Angeles smog warning system. A first alert is called when ozone intensity measures .5 ppm. A second alert is called at 1 ppm. and a third alert at 1.5 ppm.
Numerous first alerts have been called but no second or third alerts. However, a reading of .9 on September 13, 1955 brought the city within .1 ppm. of the second alert.
On the first-stage alert, a warning to cease all outdoor burning is issued. On the second-stage alert, "a health menace exists in a preliminary stage." At this stage, operation of all vehicles may be curtailed immediately and refineries or other operations deemed major smog contributors may be closed down. These include service stations, chemical plants, incinerators, open-hearth steel furnaces, secondary nonferrous refineries, electric arc furnaces, asphalt saturators, asphalt plants, ferrous and nonferrous foundries, rubber plants, paint and varnish manufacturing, tar heaters, and pavement burners. Also forbidden would be filling of ships, tank cars, and trucks with gasoline.
On the third-stage alert, all industry and virtually all traffic would be stopped and imposition of martial law would be permitted.
These are regulations of the Los Angeles Air Pollution Control District, empowered by state legislation.
In conjunction with these regulations, a new code for public forecasts of smog has been developed. "Each evening," according to the proclamation, "the local press, radio, and television will be given forecasts of smog conditions. One of two conditions will be forecast: SMOG GREEN: No emergency is expected. SMOG RED: Heavy smog forecast (your help needed). On SMOG RED, residents are asked to share rides, cut down on use of automobiles, not burn rubbish, and business and industry is asked to cooperate in all ways possible.
SELECTED BIBLIOGRAPHY
AIR AND WATER POLLUTION – Final Summary Report of Assembly Interim Committee on Air and Water Pollution. Published by the Assembly of the State of California. 1952. 111 pp.
AIR POLLUTION – Proceedings of the United States Technical Conference on Air Pollution. McGraw-Hill Book Company, Inc., 330 West 42nd Street, New York 18. 1952. 847 pp., illus., charts, tables. $12.50. (An indispensable source for information on technical, health, agricultural, and other aspects of air pollution.)
*AIR POLLUTION ABATEMENT MANUAL. Manufacturing Chemists' Association, Inc., 1625 Eye Street, N. W., Washington 6, D.C. 1952. 57 pp. $6. (Contains a wealth of technical information.)
AIR POLLUTION – A BIBLIOGRAPHY. Bulletin 537, Bureau of Mines, United States Department of the Interior. By S. J. Davenport and G. G. Morgis. Superintendent of Documents, U. S. Government Printing Office, Washington 25, D. C. 1954. 448 pp. $1.75. (A definitive bibliography. Arranged according to subjects, with abstracts under each subject in chronological order and alphabetically by author, Latest entries are dated 1952.)
*AIR POLLUTION CONTROL. Report prepared for Joint Subcommittee on Air Pollution of Assembly Interim Committees on Conservation, Planning and Public Parks, and Public Health. By the Bureau of Public Administration, University of California, Berkeley. Published by the Assembly of the State of California. January 1955. 60 pp.
AIR POLLUTION CONTROL – A NEW FRONTIER. Public Health Reports, July 1955. Pp. 633–661. (Twenty papers in brief from the First International Congress on Air Pollution, March 1–2, 1955, New York City. Copies of the full papers may be purchased from the Order Department, American Society of Mechanical Engineers, 29 West 39th Street, New York 18. Complete proceedings of the congress available in book form after October 1, 1955.)
AIR POLLUTION CONTROL IN THE COUNTY OF LOS ANGELES AND SIX OTHER METROPOLITAN AREAS. Prepared by Chief Administrative Office, Los Angeles County. 1954. 128 pp., mimeo. (Analysis of the problem mainly from the standpoints of law and administration.)
*THE CHEMICAL INDUSTRY FACTS BOOK. Second Edition. Manufacturing Chemists' Association, Inc., 1625 Eye Street, N.W., Washington 6. D. C. 1955. 148 pp. $1. (Statistical data on the chemical industry and the scope of its enterprises.)
*CLEAN AIR FOR CALIFORNIA. Initial report of the Air Pollution Study Project, California State Department of Public Health, 760 Market Street, San Francisco 2. March 1955. 57 pp., illus., maps, tables, charts.
GARBAGE IN THE SKY. Fortune, April 1955. Pp. 142–145, 176–178.
PUBLIC HEALTH NEWS, August 1955. Published by the New Jersey State Department of Health. (Entire issue devoted to two air pollution conferences: Public Conference on Open Burning Dumps sponsored by the Air Pollution Control Commission of the New Jersey State Department of Health, May 4, 1955; and seminar at Rutgers University sponsored by the Mid-Atlantic States Section of the National Air Pollution Control Association, April 13, 1955.)
THE SMOG PROBLEM IN LOS ANGELES COUNTY. A Report by Stanford Research Institute on Studies to Determine the Nature and Causes of Smog. Distributed in the public interest by the Western Oil and Gas Association, 510 West Sixth Street, Los Angeles 14. 1954. 134 pp., illus., charts. Copies available on request. (Detailed description of experiments and findings.)
SMOG SCRAMBLE SPANS NATION – Tons of "hot air" have been generated and released over public annoyance No. 1. Chemical and Engineering News, March 22, 1954. Pp. 1108–1113.
SMOKE CONTROL. A report of the Joint State Government Commission to the General Assembly of the Commonwealth of Pennsylvania, Session of 1951. 41 pp., tables.
SOME SCIENTIFIC ASPECTS OF THE URBAN AIR POLLUTION PROBLEM. Lauren B. Hitchcock and Helen G. Marcus. Scientific Monthly, July 1955. Pp. 10–21. (Summary of publishable papers presented at the American Association for the Advancement of Science Symposium.)
*A VOLUNTARY PROGRAM FOR AIR POLLUTION CONTROL IN THE SAN FRANCISCO BAY AREA – Initial Report. The San Francisco Bay Area Council, Inc., 130 Montgomery Street, San Francisco 4. January 1953. 32 pp., illus. $.50.
*Contain bibliographies.
Related PLANNING ADVISORY SERVICE Information Reports:
No. 20. AIR POLLUTION CONTROL. November 1950, 29 pp.
No. 32. PERFORMANCE STANDARDS IN INDUSTRIAL ZONING. November 1951. 19 pp.
No. 78. INDUSTRIAL ZONING STANDARDS, September 1955. 75 pp.
Copyright, American Society of Planning Officials, 1955.


